《Formation Mechanism of Iodinated Aromatic Disinfection Byproducts: Acid Catalysis with H2OI+》
DOI: https://doi.org/10.1021/acs.est.1c05484
Website: https://doi.org/10.1021/acs.est.1c05484
Graphical Abstract:
ABSTRACT:
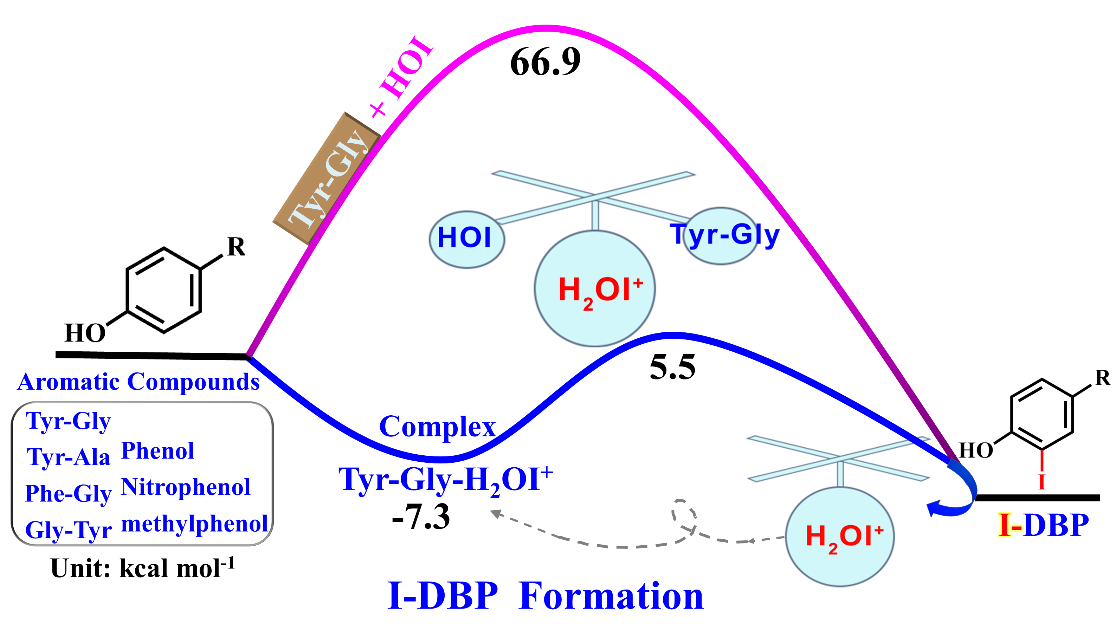
Iodinated aromatic disinfection byproducts (I-DBPs) are a group of nonregulated but highly toxic DBPs. Formation of I-DBPs is attributed mainly to HOI because it is the most abundant reactive iodine species in chloraminated water. In this study, we used computational modeling of thermodynamics to examine the mechanism of iodination of aromatic contaminants, e.g., dipeptides and phenols. Computational prediction of the energy barriers of the formation of iodinated tyrosylglycine (I-Tyr-Gly) (66.9 kcal mol-1) and hydroxylated Tyr-Gly (OH-Tyr-Gly) (46.0 kcal mol-1) via iodination with HOI favors the formation of OH-Tyr-Gly over I-Tyr-Gly. Unexpectedly, mass spectrometry experiments detected I-Tyr-Gly but not OH-Tyr-Gly, suggesting that I-Tyr-Gly formation cannot be attributed to HOI only. To clarify this question, we examined the thermodynamic role of the most reactive iodine species H2OI+ in the formation of aromatic I-DBPs under chloramination. Computational modeling of thermodynamics results shows that a loosely bonded complex formation of aromatic compounds with H2OI+ is the key step to initiate the iodination process. When H2OI+ serves as acid-catalyst and iodinating agent, with HOI or H2O acting as proton acceptor, the energy barrier of I-DBP formation was significantly lower (10.8-13.1 kcal mol-1). Therefore, even with its low concentration H2OI+ can involve in the formation of I-DBPs. These results provide insight into the mechanisms of aromatic I-DBPs formation and important information for guiding research toward controlling I-DBPs in drinking water.