《Contribution of reaction of atmospheric amine with sulfuric acid to mixing particle formation from clay mineral》

Website:https://doi.org/10.1016/j.scitotenv.2022.153336
Graphical Abstract:
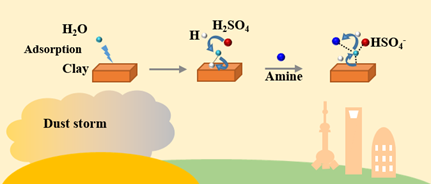
Abstract:
During dust storm, mineral particle is frequently observed to be mixed with anthropogenic pollutants (APs) and forms mixing particle which arises more complex influences on regional climate than unmixed mineral particle. Even though mixing particle formation mechanism received significant attention recently, most studies focused on the heterogeneous reaction of inorganic APs on single composition of mineral. Here, the heterogeneous reaction mechanism of amine (a proxy of organic APs) with sulfuric acid (SA) on kaolinite (Kao, a proxy of mineral dust), and its contribution to mixing particle formation are investigated under variable atmospheric conditions.Two heterogeneous reactions of Kao-SA-amine and Kao-H2O-SA-amine in absence/presence of water were comparably investigated using combined theoretical and experimental methods, respectively. The contribution from such two heterogeneous reactions to mixing particle formation was evaluated, respectively, exploring the effect of methyl groups (1−3 -CH3), relative humidity (RH) (11−100%) and temperature (220−298.15 K). Water was observed to play a significant role in promoting heterogeneous reaction of amines with SA on Kao surface, reducing formation energy of mixing particle containing ammonium salt converted by SA. Moreover, the promotion effect from water is enhanced with the increasing RH and the decreasing temperature. For methylamine and dimethylamine containing 1-2 -CH3, the heterogeneous reaction of Kao-H2O-SA-amine contributes more to mixing particle formation. However, for trimethylamine containing 3 -CH3, the heterogeneous reaction of Kao-SA-amine is the dominant source to mixing particle formation. For mixing particle generated from the above two heterogeneous reactions, ammoniums salts are supposed to be predominant components which is of strong hygroscopicity and further leads to significant influence on climate by altering radiative forcing of mixed particle and participating in the cloud condensation nuclei and ice nuclei.