Professor Jiangyao Chen and Associate Professor Weina Zhang etc. published the latest academic paper titled "Oxygen Isotope Tracing Study to Directly Reveal the Role of O2 and H2O in the Photocatalytic Oxidation Mechanism of Gaseous Monoaromatics" in ES&T
Release date:2021-11-19 Author: Source:
Click:
《Oxygen Isotope Tracing Study to Directly Reveal the Role of O2 and H2O in the Photocatalytic Oxidation Mechanism of Gaseous Monoaromatics》

Graphical Abstract:
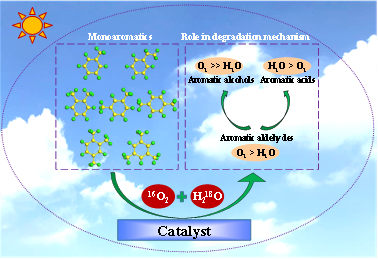
Website: https://doi.org/10.1021/acs.est.1c05134
Abstract:
O2 and H2O influence photocatalytic oxidation mechanism of gaseous monoaromatics, but still in unclear manner, due to lack of direct evidence. Tracing oxygen atom from 16O2 and H218O to intermediate can clarify their roles. Low H218O content suppressed formation of benzenedicarboxaldehydes during oxidation of xylenes and 16O2 more affected yield of total intermediates, while neither of them altered amount percentage order of products. Methylbenzaldehydes, methylbenzyl alcohols and benzenedicarboxaldehydes possessed dominant 16O percentage (≥69.49%), while higher 18O distribution was observed in methylbenzoic acids and phthalide (≥59.51%). Together with interconversion results of products revealed 16O2 determining transformation of xylenes initially to methylbenzaldehydes and then to methylbenzyl alcohols or benzenedicarboxaldehydes, while H218O mainly contributed for conversion of methylbenzaldehydes to methylbenzoic acids or phthalide. Further interaction sites of xylene and its product with H2O and O2 were confirmed by molecular dynamic calculations. Same roles of 16O2 and H218O in degradation of toluene, ethylbenzene, 1,2,4-trimethylbenzene and 1,3,5-trimethylbenzene were also verified. This is the first time to provide the direct evidence to reveal role of O2 and H2O in photocatalytic oxidation mechanism of gaseous monoaromatics. The findings are helpful to achieve controllable product formation from oxidation of monoaromatics and predict their migration process in atmospheric environment.
|
|
|
|
|
|