《A possible unaccounted source of nitrogen-containing compound formation in aerosols: amines reacting with secondary ozonides》

Website: https://doi.org/10.5194/acp-24-155-2024
Graphical Abstract:
Abstract:
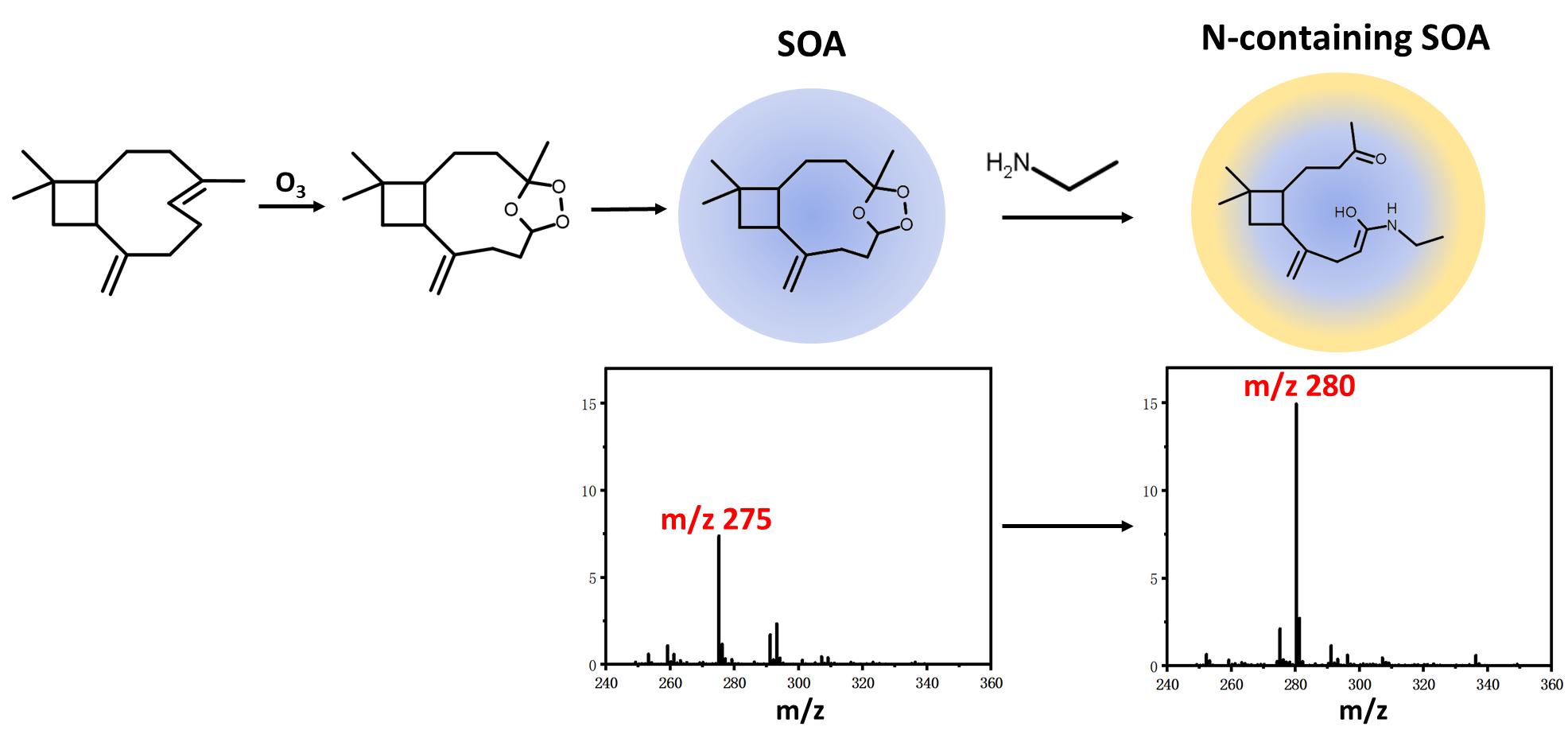
Nitrogen (N)-containing compounds have a significant impact on the optical and toxicological properties of aerosols. 1,2,4-Trioxolanes, known as secondary ozonides (SOZs), i.e., key products from the ozonolysisof biogenic terpenoids, are readily taken up into atmospheric aerosols and act as oxidants, potentially interacting with amines in the atmosphere. In the present work, we carefully investigated the component of the particles produced by the ozonolysis of β-caryophyllene (β-C) in the presence of ethylamine (EA), methylamine (MA), dimethylamine (DMA), or ammonia. The mass spectrometric results show that SOZ is the dominant product from the ozonolysis of β-C. It readily reacts with EA and MA but has inert reactivities toward DMA and ammonia. Similar experimental results were achieved with α-humulene (α-H), an isomer of β-C, was used in place of β-C. Additionally, D2O and H182O solvents were used for the characterization of products. The results revealed an intriguing phenomenon where the products from β-C SOZ and α-H SOZ reacting with the same amine (EA or MA) possessed different functional groups, despite the fact that they are isomerized species with identical chemical structure (1,2,4-trioxolane). This indicates that the chemical conformation of SOZs has a strong influence on how they react with amines. For the first time, SOZs derived from β-C and α-H reacting with amines are reported in this study; this may represent a hitherto unrecognized source of N-containing compound production in atmospheric aerosols.