《The association between estrogenic activity evolution and the formation of different products during the photochemical transformation of parabens in water》

DOI:https://doi.org/10.1016/j.watres.2025.123236
Graphical Abstract:
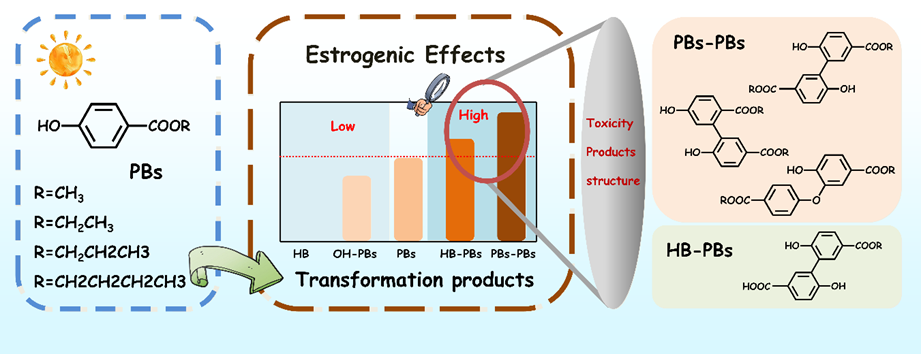
ABSTRACT:
Photochemical transformation is a critical factor influencing the environmental fate of pharmaceutical and personal care products in aquatic ecosystems. However, the relationship between toxicity evolution and the formation of various transformation products has been seldom explored. This study investigates the behavior and changes in estrogenic activity during the photochemical transformation of a series of typical endocrine-disrupting parabens (PBs), focusing on the effects of increasing alkyl-chain length (MPB, EPB, PPB and BPB). Based on MS/MS analysis, four types of transformation products were identified: (1) p-hydroxybenzoic acid (HB), which exhibits no estrogenic activity; (2) hydroxylated products (OH-PBs); (3) dimer products formed between HB and PBs (HB-PBs); and (4) dimer products formed from identical PBs (PBs-PBs), comprising three distinct isomers. In the absence of standard sample, OH-PBs were synthesized and their estrogenic activity was evaluated using a yeast two-hybrid reporter assay. The EC50 values were determined to be <1×10-3 M for OH-MPB, 2.05×10-4 M for OH-EPB, 5.05×10-5 M for OH-PPB, and 1.89×10-5 M for OH-BPB. These indicate that the estrogenic activity of OH-PBs is one order of magnitude lower than that of the corresponding PBs. Both HB-PBs and the three isomers of PBs-PBs exhibited significantly higher estrogenic activities than their corresponding parent compounds, increasing 9 – 14 and 32 − 184 times, respectively, based on theoretical calculations. Among the three PBs-PBs isomers, the highest estrogenic activity was observed in the ether dimer, followed by the biphenyl dimers. Consistent with the parent compounds, the estrogenic activities of OH-PBs, HB-PBs, and PBs-PBs increased with the length of the alkyl-chain. The estrogenic activity of MPB and EPB followed an overall downward trend during the photochemical transformation, whereas PPB and BPB remained stable initially before declining rapidly. This behavior was associated with the contributions of toxic transformation products. These findings elucidate the relationship between molecular structure, transformation products, and estrogenic activity, highlighting the importance of understanding estrogenic activity evolution during the photochemical transformation of PBs.